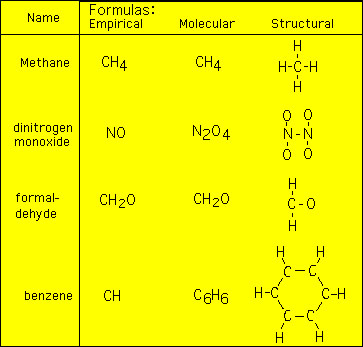
Empirical Formula of Benzene
SUBMIT ANDWER 16 Periodic Table part 1 of 3 An oxide of phosphorous. The ratio of empirical formula is.

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals
It represent the actual number of atoms of all the elements present in a compound.
. The empirical formula is the smallest whole number ratio of the number of atoms present in the element of the given compound. What is the ratio of empirical formula to molecular formula of benzene. The empirical formula is the lowest possible whole number of the ratio between the atoms of elements in the compound.
What is the ratio of empirical formula to molecular formula of benzene. The empirical formula of benzene is CH its chemical formula is C6H6. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.
Now empirical formula is the simplest ratio of a compounds constituent atoms. Answer 1 of 7. And the molecular formula for benzene which is now going to give us more information than the empirical formula tells us that each benzene molecule has six.
The ratio of empirical formula is. The correct option is A 1. The empirical formula of benzene eqC_6H_6 eq is CH.
What is the ratio of empirical formula to molecular formula of benzene. Thus the empirical formula of Benzene. Benzene is an organic compound that.
Chemistry questions and answers. Since the empirical formula is the lowest whole number formula the empirical formula for C6H6 would be CH divide both C and H by 6. What is the empirical formula of glucose and benzene.
N The molecular formula of benzene is C6H6. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms. What is the empirical formula of benzene.
Now empirical formula is the simplest ratio of a compounds constituent atoms. 6Molecular formula of benzene C6H6Molecular formula mass of benzene 78Empirical formula of benzene CHEmpirical formula mass of benzene 13. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.
Answer in units of g. Benzene C6H6 CID 241 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. The molecular formula is the representation of the actual whole number ratio between the elements of the compound.
To answer this lets see what an empirical formula is. The ratio of empirical formula is. Am empirical formula is a formula containing the whole number ratio of elements present in a compound or a molecule.
Alkene have a general formula CnH2n therefore the formula of benzene is C4H8. Distinguish between geometric and. Answer 1 of 3.
As we known that empirical formula shows the simplest whole number ratio of the atoms. Empirical formula of a chemical compound is a representation of. What is the ratio of empirical formula to molecular formula of benzene.
Molecular formula of the benzene is C6H6 Emperical formula. The molecular formula of a benzene is C_6H_6. In this case the ratio is 48 which can be simplified to 12.
What is the mass of 1 mole of the empirical formula of benzene. Thus the molar mass of benzene will be 6left 12 right 6left 1 right 78 The empirical formula is the simplest ratio of. Glucose C6H12O6 Is c2h6 an empirical.
Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.
What Is Empirical Formula And How Is It Calculated Psiberg
What Is The Empirical Formula For Benzene C6h6 Quora

The Empirical Formula Of Benzene And Acetylene Is Are Youtube

Aromatic Compounds And Electrophilic Aromatic Substitution Concise Medical Knowledge Organic Chemistry Benzene Chemistry Lessons

Comments
Post a Comment